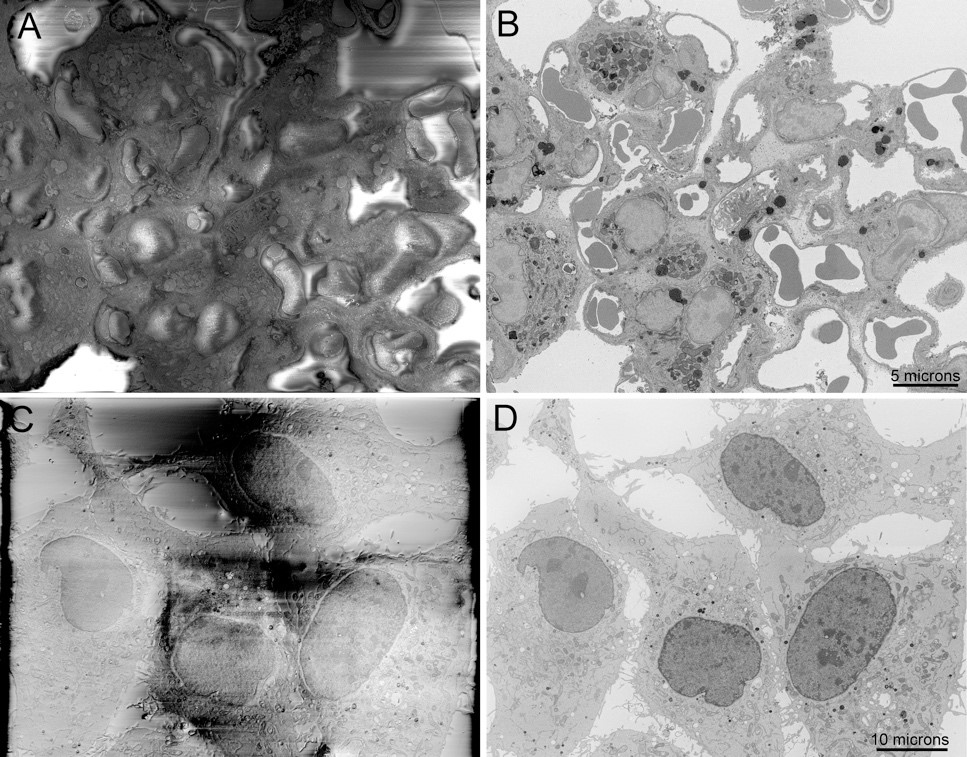
Block-face imaging of charge-prone samples of A) lung tissue and C) a cell culture monolayer with DNA stained and imaged at high vacuum, both showing major specimen charging artifacts. B and D) Complete abrogation of specimen charging of the same fields of view by focal nitrogen gas injection. Scale bars = 5 and 10 microns.
Electron microscopy has played an integral role in our understanding of a wide range of neurological diseases, including AD. SBEM is a rapidly proliferating technique that allows for the imaging and reconstruction of large expanses of brain tissue (tens of thousands of cubic microns) at nanometer-scale resolution.30-32 SBEM consists of a computer-controlled ultramicrotome fitted into a scanning electron microscope. With SBEM, an oscillating diamond knife removes a very thin (20-100 nm) layer from the epoxy embedded sample surface, which is then imaged using backscattered electrons. This process is repeated hundreds or even thousands of times sequentially in an automated manner until the desired volume of tissue is traversed.
Until very recently, one of the major limitations of this approach was that either variable-pressure SEM (VP-SEM) or special enhanced heavy metal staining must be used to mitigate specimen charging that results from requisite embedding of the specimen within non-conductive epoxy resin. With VP-SEM, gas molecules are introduced into the specimen chamber and the resulting ions formed by electron-gas interactions serve to dissipate surface charging. Unfortunately, this results in a tremendous reduction in signal-to-noise ratio (SNR) and resolution due to electron scattering from interactions with the gas molecules. As an alternative, we introduced intense heavy metal staining of tissues for SBEM that makes it possible to image many specimens, especially brain tissue, at high vacuum, dramatically improving SNR and resolution.8 Unfortunately, specimens that were prepared without this approach still required the use of VP-SEM.
Recognizing the limitations of these approaches, we recently developed and introduced a new approach to SBEM that uses focal nitrogen gas-injection over the sample block face surface to eliminate sample charging while allowing high vacuum to be maintained in the specimen chamber, resulting, once again, in a significant improvement in both SNR and resolution, but now for specimens not intensely stained with heavy metals. We refer to this approach as Focal Charge Compensation (Focal CC)
By and large, our brain biopsy samples from AD patients were prepared using conventional EM fixation, staining and processing protocols and did not employ the multiple en bloc heavy metal staining protocol developed by us to propel high-resolution SBEM. With the advent of Focal CC, however, we have removed the need to use strong metal staining, allowing for larger-scale reanalysis of these samples. As a validation, we applied the Focal CC enhanced SBEM method to the samples from this collection, determining that excellent image quality can be obtained with resolution sufficient to resolve microtubules and synaptic vesicles.